Common Problems and Selection of Additives
Common problems during molding and production processes and the corrective measures are explained below. Please refer to the information below when selecting additives.
Frequently Asked Questions
-
Discoloration
Q9
Why does NOx discoloration occur?
A9
 Discolored plastic materials are often found in warehouses and along roads with a large amount of vehicle traffic, etc. The NOx gas in exhaust gases is said to cause oxygenation of phenolic antioxidants, which results in discoloration.
Discolored plastic materials are often found in warehouses and along roads with a large amount of vehicle traffic, etc. The NOx gas in exhaust gases is said to cause oxygenation of phenolic antioxidants, which results in discoloration.
Corrective Measures
- 1
- Use a phenolic antioxidant with high resistance to discoloration (ADK STAB AO-80, etc.).
- 2
- Replace the phosphorus antioxidant used with one which is more reactive (ADK STAB PEP-36, etc.).
- 3
- Increase the amount of phosphorus antioxidant.Phosphorus Antioxidant
Q10
Why does “yellowing in the dark” occur?
A10
Polymers such as ABS without pigments may discolor yellowish in the dark. Although its mechanism is still not clearly understood, this phenomenon is believed to proceed due to the presence of radical species which are produced by thermal oxidation resulting from heat exposure during processing or by initial photo-oxidation.
Corrective Measures
- 1
- Enhance stability during processing by using antioxidants (ADK STAB AO-80, PEP-36, etc.).
- 2
- Protect the plastic from initial photo-oxidation by using UVA(ADK STAB LA-31, LA-36, etc.). Ultraviolet Absorber (UVA)
- 3
- Scavenge the radical species by using HALS (ADK STAB LA-52, LA-72, LA-81, etc.). Light Stabilizer (HALS)
Q11
Why does light discoloration occur?
A11-1
 In the case of polyvinyl chlorides (PVC):
As part of the degradation process, PVC undergo dehydrochlorination, which increases the number of polyene conjugated double bonds etc., resulting in discoloration.
In the case of polyvinyl chlorides (PVC):
As part of the degradation process, PVC undergo dehydrochlorination, which increases the number of polyene conjugated double bonds etc., resulting in discoloration.
Corrective Measures
- 1
- Use an appropriate stabilizer capable of quick stabilization of the PVC following dehydrochlorination.
A11-2
In the case of polyolefins (polypropylene and polyethylene ): When polyolefins are exposed to heat or light, hydrogen atoms in their structure are withdrawn, and the polyolefins are degraded through an autoxidation reaction. This degradation process produces polyene, resulting in discoloration.
Corrective Measures
- 1
- Enhance stabilization during processing by using phosphorus antioxidants capable of preventing thermo-oxidative degradation.
- 2
- Blend in a light stabilizer (such as a HALS or UVA), thereby preventing photo-degradation.
A11-3
In the case of polycarbonate (PC): PC absorbs ultraviolet light of wavelength approximately 290 nm and undergoes the photo-reaction known as the Fries rearrangement. This reaction produces compounds with phenyl salicylate and dihydroxy benzophenone structures, resulting in yellowing.
Corrective Measures
- 1
- Add an Ultraviolet Absorber (UVA). One effective method is to form a surface coat consisting of a polymer that contains a high concentration of UVA, or to incorporate UVA into a hard coating. Ultraviolet Absorber (UVA)
- 2
- Benzotriazole and triazine UVA are effective types.
Q12
Why are plastics discolored by fillers and pigments?
A12
 Additives that have a phenolic group, such as phenolic antioxidants and ultraviolet absorbers, may form a chelate with the metal ions contained in fillers or pigments, resulting in coloration.
Additives that have a phenolic group, such as phenolic antioxidants and ultraviolet absorbers, may form a chelate with the metal ions contained in fillers or pigments, resulting in coloration.
Corrective Measures
- 1
- Scavenge the metal ions by using a Metal Deactivator (ADK STAB CDA-1, etc.). Metal Deactivator
- 2
- Replace the UVA with a triazine type or stop using.
Q13
Why are plastics discolored by phenol?
A13
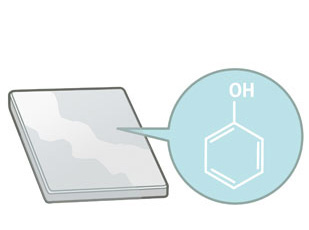 Phenolic antioxidants often cause discoloration because they are oxidized to form colored quinone compounds. The same may also occur in polymers that decompose to produce a phenolic group.
Phenolic antioxidants often cause discoloration because they are oxidized to form colored quinone compounds. The same may also occur in polymers that decompose to produce a phenolic group.
Corrective Measures
- 1
- Phosphorus antioxidants (ADK STAB PEP-36 etc.) are effective for preventing discoloration during processing.
- 2
- For polymers that decompose to produce a phenolic group, use a phosphorus antioxidant to enhance stabilization during processing. If the polymer has a strong tendency to hydrolyze, moisture should be removed before processing. In addition, when the polymer is used, UVA should be blended into it in order to prevent photodecomposition.
